Levothyroxine, the synthetic form of thyroxine hormone, serves as a cornerstone treatment for hypothyroidism, affecting millions of patients worldwide. While this medication proves highly effective in restoring normal thyroid function, a subset of patients experience distressing cutaneous reactions that can significantly impact their quality of life. These skin manifestations range from mild urticaria to severe dermatological conditions, creating diagnostic challenges for healthcare professionals and forcing patients to consider alternative treatment approaches. Understanding the underlying mechanisms behind levothyroxine-induced skin reactions becomes crucial for both patients and clinicians navigating thyroid hormone replacement therapy. The complexity of these reactions stems not only from the active pharmaceutical ingredient but also from various excipients and manufacturing components that can trigger hypersensitivity responses in susceptible individuals.
Levothyroxine-induced cutaneous hypersensitivity reactions: pathophysiology and mechanisms
The pathophysiology of levothyroxine-induced skin reactions involves multiple immunological pathways, each contributing to distinct clinical presentations. These reactions occur despite levothyroxine being a synthetic replica of naturally occurring thyroid hormone, highlighting the complex interplay between medication formulation and individual immune responses. The mechanisms underlying these reactions provide insight into why some patients develop adverse cutaneous effects while others tolerate the medication without incident.
Type IV delayed hypersensitivity response to synthetic T4 hormone
Type IV delayed hypersensitivity represents one of the primary mechanisms behind levothyroxine-induced skin reactions. This T-cell mediated response typically manifests 24-72 hours after drug exposure, creating a characteristic pattern of eczematous dermatitis. The synthetic T4 hormone acts as a hapten, binding to carrier proteins and creating novel antigenic complexes that trigger sensitised T-lymphocytes. Memory T-cells recognise these modified protein structures as foreign, initiating a cascade of inflammatory mediators including interferon-gamma and tumour necrosis factor-alpha. This delayed response explains why patients may tolerate levothyroxine for weeks or months before developing skin symptoms, as the sensitisation process requires repeated exposure to establish immunological memory.
Cross-reactivity between levothyroxine sodium and excipient components
Cross-reactivity patterns between levothyroxine sodium and various excipients create complex allergic responses that can be difficult to distinguish from reactions to the active ingredient itself. Pharmaceutical excipients such as lactose monohydrate, microcrystalline cellulose, and various binding agents share structural similarities that can trigger cross-reactive immune responses. This phenomenon becomes particularly problematic when patients attempt to switch between different levothyroxine formulations, as they may continue experiencing allergic reactions despite changing the brand. The molecular mimicry between excipient components and the levothyroxine molecule itself can perpetuate hypersensitivity reactions even when the primary allergen has been identified and avoided.
Ige-mediated immediate hypersensitivity in levothyroxine allergy
IgE-mediated immediate hypersensitivity reactions to levothyroxine, while less common than delayed responses, can produce severe and potentially life-threatening cutaneous manifestations. These reactions typically occur within minutes to hours of drug administration, involving mast cell degranulation and histamine release. The rapid onset of urticaria, angioedema, and bronchospasm characterises this type of hypersensitivity response. Patients experiencing IgE-mediated reactions often demonstrate elevated specific IgE antibodies against levothyroxine or associated excipients through laboratory testing. The severity of these reactions necessitates immediate discontinuation of the medication and careful consideration of alternative thyroid hormone replacement strategies.
Complement activation pathways in thyroid hormone replacement therapy
Complement activation pathways contribute to the inflammatory cascade observed in levothyroxine-induced skin reactions through both classical and alternative routes. The classical pathway activation occurs when antibody-antigen complexes formed between levothyroxine and specific immunoglobulins trigger C1 complex activation. Alternative pathway activation can occur independently of antibodies, involving direct interaction between levothyroxine formulation components and complement proteins. This dual activation mechanism results in the production of anaphylatoxins C3a and C5a, which increase vascular permeability and promote inflammatory cell recruitment to affected skin areas. The complement-mediated inflammation contributes to the erythema, oedema, and pruritus commonly observed in patients experiencing cutaneous reactions to levothyroxine therapy.
Brand-specific allergenic components in levothyroxine formulations
Different levothyroxine manufacturers utilise varying excipient profiles, creating distinct allergenic potential across brand formulations. Understanding these brand-specific components becomes essential for patients who experience adverse reactions, as switching between formulations may resolve symptoms or, conversely, introduce new allergens. The pharmaceutical industry’s approach to excipient selection prioritises stability and bioavailability, sometimes inadvertently incorporating components that trigger hypersensitivity reactions in susceptible individuals.
Synthroid lactose monohydrate and acacia sensitivity reactions
Synthroid, one of the most prescribed levothyroxine formulations, contains lactose monohydrate and acacia as primary excipients, both known to trigger allergic reactions in sensitive individuals. Lactose intolerance affects approximately 65% of the global population, and while most reactions involve gastrointestinal symptoms, cutaneous manifestations can occur in severely sensitive patients. Acacia, derived from the Acacia senegal tree, represents a potent allergen capable of inducing both immediate and delayed hypersensitivity reactions. Patients sensitive to acacia may develop urticaria, contact dermatitis, or respiratory symptoms within hours of Synthroid administration. The combination of these two allergens in a single formulation creates particular challenges for patients with multiple sensitivities who require thyroid hormone replacement therapy.
Levoxyl microcrystalline cellulose and magnesium stearate allergenicity
Levoxyl utilises microcrystalline cellulose and magnesium stearate as key excipients, presenting different allergenic profiles compared to other levothyroxine formulations. Microcrystalline cellulose, while generally considered hypoallergenic, can trigger reactions in patients with severe cellulose sensitivities or those with concurrent allergies to plant-derived materials. Magnesium stearate, commonly used as a pharmaceutical lubricant, has been implicated in contact sensitisation reactions, particularly in individuals with existing metal allergies. The particle size and processing methods used in Levoxyl manufacturing can influence the bioavailability of these excipients, potentially affecting their allergenic potential. Patients switching from other formulations to Levoxyl may experience resolution of previous allergic symptoms, though new sensitivities can emerge with continued exposure.
Tirosint gel cap glycerin and gelatin hypersensitivity manifestations
Tirosint’s gel capsule formulation presents unique allergenic challenges through its glycerin and gelatin components, particularly problematic for patients with religious dietary restrictions or animal protein sensitivities. Gelatin, derived from bovine or porcine sources, can trigger severe allergic reactions in individuals with alpha-gal syndrome or those with pre-existing meat allergies. The glycerin component, while typically well-tolerated, can cause contact dermatitis in sensitive individuals, particularly those with concurrent sensitivities to propylene glycol or other polyols. The gel capsule delivery system may also affect the rate of allergen exposure, potentially intensifying reactions in susceptible patients. Despite being marketed as a “cleaner” formulation with fewer excipients, Tirosint can still produce significant allergic reactions in appropriately sensitised individuals.
Generic levothyroxine dye additives: tartrazine and FD&C colorants
Generic levothyroxine formulations often incorporate various dye additives, including tartrazine (Yellow No. 5) and other FD&C colorants, which represent significant allergens for sensitive patients. Tartrazine sensitivity affects approximately 0.1% of the general population but shows increased prevalence among individuals with aspirin sensitivity and asthma. These synthetic colorants can trigger both immediate urticarial reactions and delayed eczematous responses, creating diagnostic confusion when patients experience symptoms hours to days after medication administration. The colour-coding system used to differentiate levothyroxine dosage strengths necessitates the use of these dyes, creating unavoidable exposure for patients requiring specific dosages. Generic manufacturers may utilise different dye combinations compared to brand formulations, making it challenging to identify the specific allergenic component responsible for adverse reactions.
Dermatological manifestations of levothyroxine intolerance
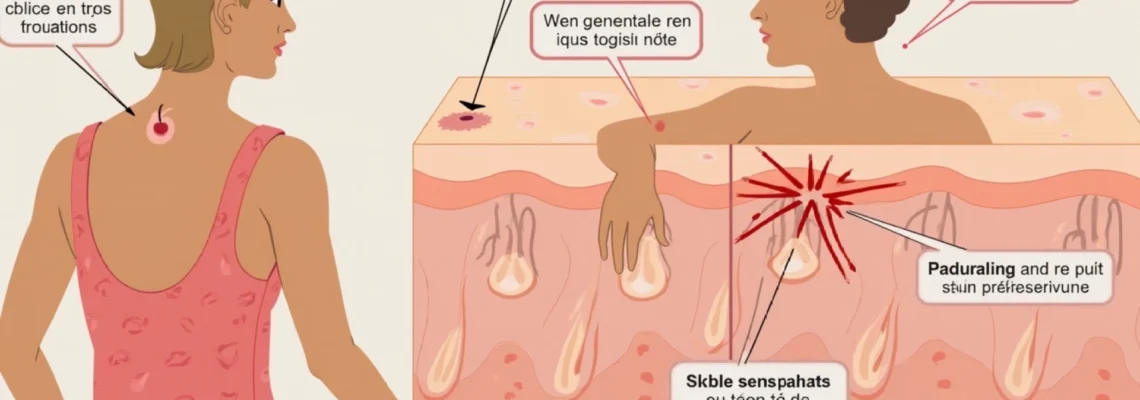
The cutaneous manifestations of levothyroxine intolerance encompass a broad spectrum of dermatological conditions, ranging from mild, localised skin irritation to severe, systemic hypersensitivity reactions. These reactions can significantly impact patient compliance with thyroid hormone replacement therapy and may necessitate alternative treatment approaches. Understanding the various presentation patterns helps clinicians differentiate between true allergic reactions and other adverse effects, enabling appropriate management strategies.
Urticarial eruptions and angioedema following oral administration
Urticarial eruptions represent the most commonly reported cutaneous reaction to levothyroxine, typically manifesting as raised, erythematous wheals accompanied by intense pruritus. These lesions often appear within 30 minutes to 6 hours after medication administration, suggesting an IgE-mediated hypersensitivity mechanism. The wheals may be localised to specific body regions or generalised across the entire integument, with severity correlating to the degree of sensitisation. Angioedema frequently accompanies urticarial reactions, particularly affecting the periorbital region, lips, and tongue. This deeper dermal and subcutaneous swelling can pose serious complications when involving the larynx or pharynx, potentially requiring emergency medical intervention. The transient nature of urticarial lesions, typically resolving within 24 hours, distinguishes them from other chronic dermatological conditions.
The unpredictable timing of urticarial reactions creates additional challenges for patients and clinicians attempting to establish causality between levothyroxine administration and symptom onset. Some patients report delayed urticarial responses occurring 12-24 hours post-administration, suggesting complex immune mechanisms beyond simple IgE-mediated responses. The intensity of pruritus associated with levothyroxine-induced urticaria often proves more severe than that seen with other drug-induced reactions, significantly impacting sleep quality and daily activities.
Eczematous dermatitis and contact sensitisation patterns
Eczematous dermatitis following levothyroxine administration typically presents as a delayed hypersensitivity reaction, developing 48-72 hours after drug exposure. The characteristic features include erythematous, scaling patches with vesicle formation, particularly affecting areas with increased skin thickness such as the palms and soles. This presentation pattern suggests a Type IV delayed hypersensitivity mechanism involving T-cell activation and cytokine release. Patients may initially experience mild erythema and pruritus, which progressively develops into more pronounced eczematous changes with continued medication exposure.
Contact sensitisation patterns can also develop through handling of levothyroxine tablets, particularly in healthcare workers or patients who frequently manipulate their medication. The powder residue from tablet handling can create localised contact dermatitis on fingertips and hands, which may progress to more widespread eczematous dermatitis with continued exposure. This occupational sensitisation highlights the importance of proper medication handling techniques and the potential for developing systemic sensitivity through localised contact exposure.
Erythema multiforme and Stevens-Johnson syndrome risk factors
Erythema multiforme represents a more severe manifestation of levothyroxine hypersensitivity, characterised by distinctive target-shaped lesions predominantly affecting the extremities. These lesions typically measure 1-3 centimetres in diameter and display the pathognomonic three-zone appearance with a central dusky area, surrounding pale zone, and peripheral erythematous ring. The development of erythema multiforme following levothyroxine administration suggests a severe immunological response requiring immediate drug discontinuation and supportive care.
Stevens-Johnson syndrome, while extremely rare with levothyroxine therapy, represents the most severe end of the cutaneous reaction spectrum. This life-threatening condition involves widespread epidermal necrosis and mucosal involvement, requiring intensive care management. Risk factors for developing severe cutaneous reactions include previous drug allergies, concurrent medication use, and genetic predisposition factors such as specific HLA allotypes. The rarity of these severe reactions should not diminish the importance of recognising early warning signs and implementing appropriate management strategies to prevent progression to life-threatening complications.
Chronic pruritus and lichenification in Long-Term users
Chronic pruritus affecting long-term levothyroxine users often develops insidiously, initially dismissed as dry skin or unrelated dermatological conditions. This persistent itching can occur without visible skin changes initially, creating diagnostic challenges for healthcare providers. The pruritus typically follows a pattern of worsening approximately 2-4 hours after medication administration, providing an important temporal clue for diagnosis. Patients may report that the itching interferes with sleep and daily activities, significantly impacting quality of life.
Lichenification develops as a consequence of chronic scratching and rubbing in response to persistent pruritus. The affected skin becomes thickened, with accentuated skin markings and a leathery texture, particularly affecting easily accessible areas such as the forearms, shins, and neck. This secondary change indicates chronic inflammation and mechanical trauma, requiring both allergen avoidance and symptomatic treatment of the lichenified areas. The development of lichenification suggests prolonged exposure to the allergenic stimulus, emphasising the importance of early recognition and intervention in levothyroxine-induced cutaneous reactions.
Diagnostic approaches for Levothyroxine-Related cutaneous reactions
Diagnosing levothyroxine-related cutaneous reactions requires a systematic approach combining clinical assessment, temporal correlation, and specialised testing when appropriate. The challenge lies in distinguishing true allergic reactions from other adverse effects or concurrent dermatological conditions. Healthcare providers must consider the complex interplay between active pharmaceutical ingredients and various excipients when evaluating patients with suspected levothyroxine hypersensitivity. The diagnostic process becomes particularly complex given the essential nature of thyroid hormone replacement therapy and the potential consequences of unnecessary drug discontinuation.
Clinical history remains the cornerstone of diagnosis, requiring detailed documentation of symptom onset, duration, and relationship to medication administration. Patients should maintain detailed symptom diaries recording the timing of levothyroxine intake, onset of cutaneous symptoms, and any concurrent medications or exposures. The temporal relationship between drug administration and symptom development provides crucial diagnostic information, with immediate reactions suggesting IgE-mediated hypersensitivity and delayed reactions indicating T-cell mediated responses. Photography of cutaneous lesions during acute episodes can provide valuable documentation for healthcare providers, particularly when patients present during symptom-free intervals.
Patch testing represents the gold standard for diagnosing delayed-type hypersensitivity reactions to levothyroxine and associated excipients. This procedure involves applying small amounts of suspected allergens to the patient’s back under occlusive patches, with readings performed at 48 and 96 hours post-application. The testing panel should include both the active ingredient levothyroxine sodium and relevant excipients based on the patient’s medication history. However, standardised patch test concentrations for levothyroxine components remain limited, often requiring customised preparations based on clinical experience and published case reports.
Skin prick tests and intradermal testing may be considered for patients with suspected immediate hypersensitivity reactions, though these carry inherent risks of triggering severe systemic reactions and should only be performed in appropriate clinical settings with emergency management capabilities.
Laboratory investigations can provide supportive evidence for allergic reactions, though specific tests for levothyroxine hypersensitivity remain limited. Total IgE levels may be elevated during acute allergic episodes, while specific IgE testing for levothyroxine is not commercially available in most laboratories. Tryptase levels can be measured during acute urticarial reactions to assess mast cell activation, providing evidence for IgE-mediated reactions when elevated within 1-4 hours of symptom onset. Complete blood count with differential may reveal eosinophilia in patients with drug-induced hypersensitivity syndrome, though this finding is not specific to levothyroxine reactions.
Provocation testing, while potentially definitive, carries significant risks and should only be considered in specialised allergy centres with appropriate resuscitation facilities. Oral challenge tests with incremental doses of levothyroxine can confirm or exclude hypersens
itivity reactions, but should be reserved for cases where alternative thyroid hormone replacement options are limited and the clinical suspicion for true allergy is high.
Alternative thyroid hormone replacement strategies for allergic patients
Patients experiencing confirmed levothyroxine hypersensitivity reactions require careful consideration of alternative thyroid hormone replacement strategies to maintain optimal thyroid function while avoiding allergenic exposures. The selection of appropriate alternatives depends on the specific allergen identified, the severity of previous reactions, and individual patient factors including age, cardiovascular status, and concurrent medical conditions. Healthcare providers must balance the need for effective thyroid hormone replacement with the imperative to avoid potentially life-threatening allergic reactions.
Liothyronine (T3) monotherapy represents a viable alternative for patients with confirmed levothyroxine hypersensitivity, particularly when reactions are specifically attributed to the T4 molecule or its synthetic formulation. This synthetic triiodothyronine preparation bypasses the need for peripheral T4 to T3 conversion, providing direct thyroid hormone activity. However, liothyronine requires more frequent dosing due to its shorter half-life, typically administered twice daily compared to once-daily levothyroxine dosing. The rapid onset and shorter duration of action can result in fluctuating thyroid hormone levels, potentially causing symptoms of both hyper- and hypothyroidism throughout the dosing interval. Patients transitioning to liothyronine monotherapy require careful monitoring and dose adjustments to achieve stable thyroid function without experiencing the peaks and valleys associated with this medication’s pharmacokinetic profile.
Natural desiccated thyroid (NDT) preparations, derived from porcine thyroid glands, offer another alternative for patients unable to tolerate synthetic levothyroxine formulations. These preparations contain both T4 and T3 in approximately a 4:1 ratio, more closely mimicking the hormone profile of healthy human thyroid glands. NDT products such as Armour Thyroid, Nature-Throid, and WP Thyroid utilise different excipient profiles compared to synthetic preparations, potentially avoiding the specific allergens responsible for previous reactions. However, patients with pork allergies or religious dietary restrictions may not be suitable candidates for NDT therapy. The biological variability inherent in animal-derived products can result in batch-to-batch potency variations, requiring more frequent monitoring compared to synthetic preparations.
Compounded thyroid hormone preparations offer highly customised solutions for patients with multiple drug sensitivities or specific excipient allergies. Specialised compounding pharmacies can prepare levothyroxine or liothyronine using hypoallergenic excipients, avoiding known allergens identified through patch testing or clinical history. These preparations can be formulated as capsules using alternative binders and fillers, or as sublingual tablets that bypass gastrointestinal absorption. The flexibility of compounded preparations allows for precise dose adjustments and the elimination of problematic colorants, preservatives, or other additives found in commercial formulations. However, compounded medications may lack the rigorous quality control and bioequivalence testing associated with FDA-approved products, potentially resulting in variable potency and absorption.
Levothyroxine liquid formulations present another option for patients with tablet or capsule excipient sensitivities, though availability remains limited in many regions. These liquid preparations typically contain fewer excipients compared to solid dosage forms, reducing the likelihood of allergic reactions in sensitised individuals. The liquid formulation allows for precise dose titration and may be particularly beneficial for pediatric patients or adults with swallowing difficulties. However, liquid levothyroxine preparations require specific storage conditions and have shorter shelf lives compared to tablet formulations, potentially creating logistical challenges for long-term therapy.
Immunosuppressive strategies may be considered in select cases where no suitable alternative thyroid hormone preparation can be identified and the patient requires continued levothyroxine therapy. Low-dose corticosteroids or antihistamine premedication protocols have been used successfully in limited case reports, though these approaches carry their own risks and require careful risk-benefit assessment. Desensitisation protocols, involving gradual reintroduction of levothyroxine with incrementally increasing doses, have shown success in selected patients with mild to moderate hypersensitivity reactions. These protocols must be conducted in supervised clinical settings with emergency management capabilities readily available.
The monitoring requirements for patients using alternative thyroid hormone replacement strategies differ significantly from standard levothyroxine therapy protocols. More frequent laboratory monitoring is typically necessary during the initial transition period, with thyroid function tests performed every 2-4 weeks until stable hormone levels are achieved. Patients using liothyronine preparations may require monitoring of both TSH and free T3 levels, as conventional TSH-based monitoring may not accurately reflect thyroid hormone status with T3 monotherapy. The complexity of alternative dosing regimens necessitates enhanced patient education regarding medication timing, food interactions, and recognition of symptoms suggesting over- or under-replacement.
Long-term outcomes for patients using alternative thyroid hormone replacement strategies generally remain favourable, though may require more intensive clinical management compared to standard levothyroxine therapy. Patients successfully transitioned to alternative preparations typically report resolution of previous allergic symptoms while maintaining adequate thyroid hormone replacement. However, the limited availability and higher costs associated with some alternative preparations can create access barriers for certain patient populations. Healthcare systems must balance the clinical necessity of avoiding allergic reactions with the practical considerations of medication availability and cost-effectiveness when supporting patients requiring alternative thyroid hormone replacement strategies.